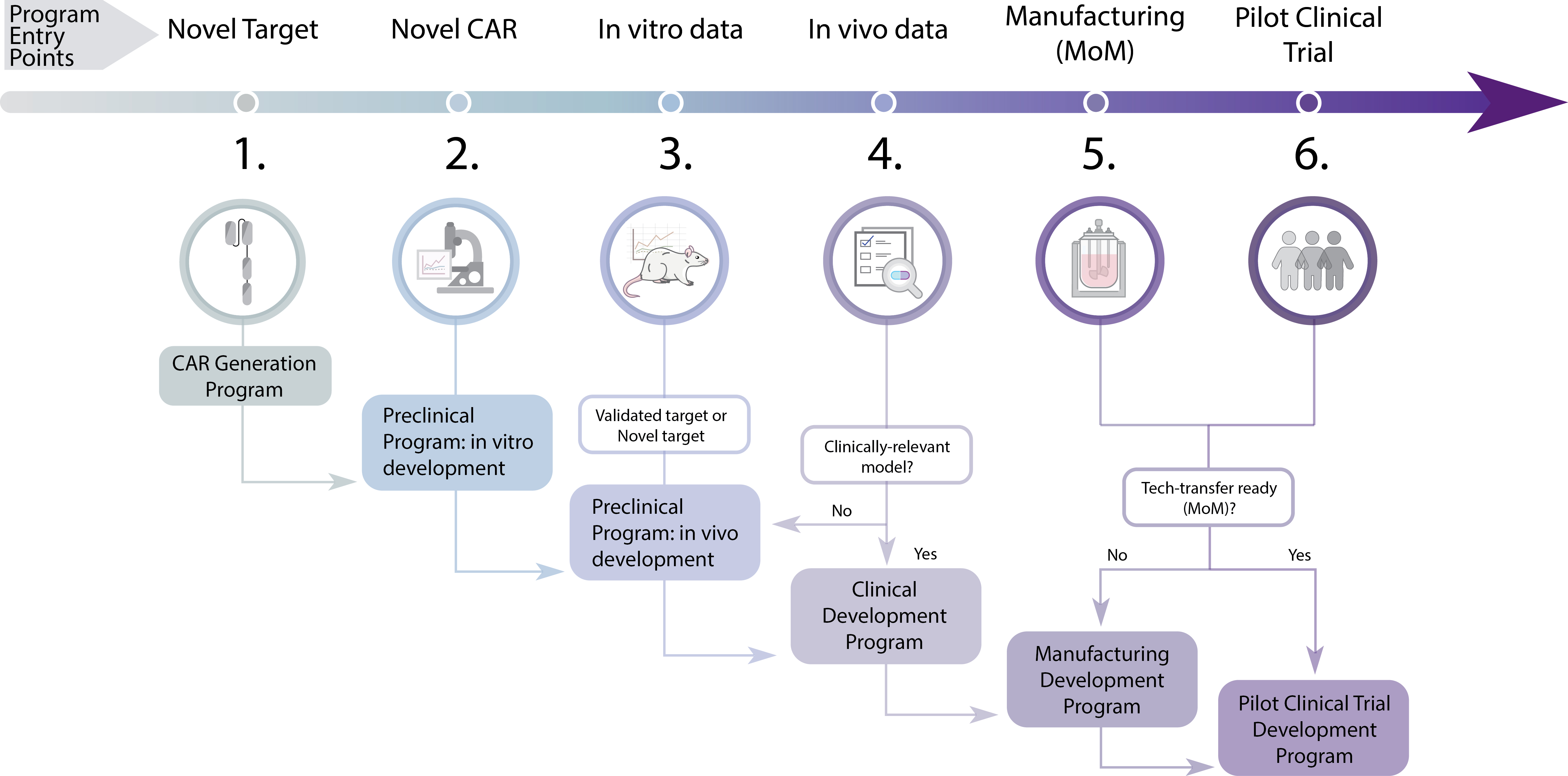
The Centre of Excellence in Cellular Immunotherapy Development Program offers end-to-end capabilities in the development of novel cellular immunotherapies. From preclinical development in our Translation Lab, through to development of GMP manufacturing processes, implementation of GMP clinical trial manufacturing and execution of a first-in-human clinical trial, the Development Program aims to fast-track the translation of novel immunotherapies.
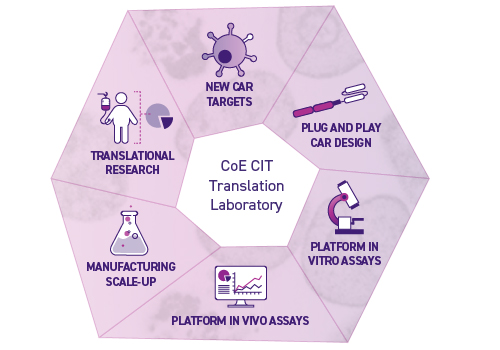
The Centre’s Translation Laboratory has extensive expertise in the performing in vitro and in vivo testing of CAR-T and other immunotherapy products to generate safety and efficacy data for regulatory packages to the TGA and HREC committees.
The Translation Laboratory team can also develop the product method of GMP manufacturing. This expedites the technology transfer process via co-located manufacturing partners, Cell Therapies Pty Ltd., minimising timelines to GMP production.
In addition, the Translation Laboratory team can assist in the following:
- CAR (Chimeric Antigen Receptor) design
- CAR-T cell generation for preclinical research
- Human and murine in vitro assays to assess CAR-T cell functionality
- Murine in vivo models to assess CAR-T cell efficacy and safety
- Translational and correlative work to support clinical trial readouts
The Development Program package includes procurement of GMP and GMP-like reagents suitable for a TGA regulated Phase I, first-in-human clinical trial and all associated quality controls.
The Centre is physically co-located with the program’s manufacturing partners, Cell Therapies Pty. Ltd. We work collaboratively to establish efficient, robust GMP manufacturing processes for CAR-T and other cellular therapy products.
Through regular interaction and engagement, processes developed in the Translation Laboratory can be seamlessly transferred to Cell Therapies cleanrooms for tech transfer and process qualification runs.

For more information on the Centre of Excellence in Cellular Immunotherapy Translational Laboratory, contact